DISCLAIMER: Active healthcare claims on treated articles
***Active healthcare claims on treated articles such as antiviral are not permitted in the USA. Antiviral claims are permitted in Germany on most treated articles and can be evaluated on a case-by-case basis in other EU territory. Please contact HeiQ for all treated article claims approval prior to commercialization.*** This web page reflects the regulatory situation in Europe. Antimicrobial properties built in to protect the product. Product does not protect users or others against pathogens. Always clean the product thoroughly after each use.

HeiQ Viroblock™ NPJ03 is an intelligent Swiss textile technology that is added to the fabric during the final stage of the textile manufacturing process.
Tested effective against SARS-CoV-2 (COVID-19)(1)
- HeiQ Viroblock™ NPJ03 has been tested effective 99.99% in 30 minutes against SARS-CoV-2, the COVID-19 causing virus(1)
- Residual virus infectivity tested according to a modified ISO 20743 method (Sendai virus), rapid antiviral effect demonstrated within 2-5 minutes(2)
- Tested according to ISO 18184 as strong antiviral and ISO 20743 as strong antibacterial against enveloped viruses and bacteria(3)
- HeiQ Viroblock™ Technology was developed at first for the Ebola crisis in 2013. The medical class 1 FFP2 masks used during the crisis in Africa have recently been tested again. The antimicrobial performance was unchanged after 7 years of shelf-life (4)
- HeiQ won the prestigious Swiss Technology Award 2020 for HeiQ Viroblock™
- OEKOTEX® suited, ZDHC and bluesign® homologized
- Treated textile articles are compliant with EU BPR
- Suitable for all fiber types, from medical nonwovens (e.g. face masks) to fabrics for clothing and home textiles
- For washable fabrics: Lasts at least 30 gentle washes at 60°C (140°F) ((ISO 6330 6G)
- Resists on wool up to 5 dry cleanings
- Provides a germ resistant surface
- Patent pending antiviral and antibacterial technology
- For antimicrobial odor control treatments used on textiles, please visit our HeiQ Pure page

(1) M.J. (2020) Report on “Viral Stability and Persistence of SARS-CoV-2 on Treated Material”. Doherty Institute for Infection and Immunity, Australia
(2) 2013, Geneva University, “Modified ISO 20743, Sendai virus contact kill time testing”, Dr. Thierry Pelet, Switzerland
(3) 2020, (6720)179-0376-1 test report, “ISO 18184 – Determination of antiviral activity of textile products”, Bureau Veritas, India ; 2020, LS20-02090 test report, “ISO 20743 – Antibacterial activity after 50 x domestic laundering (ISO 6330)”, Microbe Investigations, Switzerland
(4) 2020, LS20-01386 test report, “ISO 20743 – Antibacterial activity on face mask after 7 years on the shelf”, Microbe Investigations, Switzerland
TRADEMARKS: HeiQ, HeiQ Viroblock and Viroblock are trademarks or registered trademarks owned by HeiQ Materials AG. The use of such trademark(s) is subject to trademark licensing, review of test report and approval of claims by HeiQ. No unauthorized use permitted. Please contact us for more information.
DISCLAIMER: This web page reflects the regulatory situation in Europe. Every jurisdiction has different regulatory requirements for the marketing of treated articles. Please contact us for more information.
Tested effective against SARS-CoV-2*
99.99% reduction of the COVID-19 causing virus
HeiQ Viroblock™ NPJ03 is among the first textile technologies in the world to be proven effective against SARS-CoV-2 by an independent institution*. SARS-CoV-2 is an enveloped virus from the coronavirus family that causes COVID-19.
Tests have been conducted by HeiQ with the Peter Doherty Institute for Infection and Immunity in Melbourne, Australia (Doherty Institute).
HeiQ Viroblock™ is designed to inhibit the growth and persistence of bacteria and enveloped viruses on textile surfaces. It’s a unique combination of our registered silver technology for antiviral and antibacterial effect and our vesicle technology as a booster.
* M.J. (2020) Report on “Viral Stability and Persistence of SARS-CoV-2 on Treated Material”. Doherty Institute for Infection and Immunity, Melbourne.
Discover more about this technology!
HeiQ Viroblock™ NPJ03 treated face masks
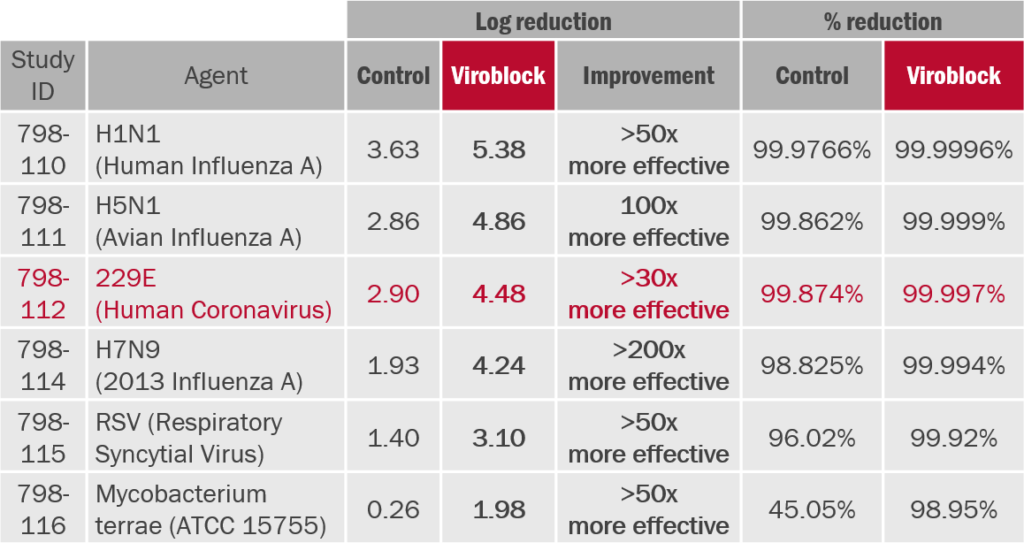
Aerosol challenge test ASTM F2101(7): Face masks (N95/FFP2/KN95 type) treated with HeiQ Viroblock™ NPJ03 (Viroblock) have been tested according to GLP standards relative to the untreated control masks.(8)
Treated face masks show significantly improved reduction in virus infectivity compared to untreated control masks.(9)
Antiviral efficacy test (ISO 18184): Nonwoven material for disposable face masks treated with HeiQ Viroblock NPJ03 has been tested according to ISO 18184 standard.(10)
HeiQ Viroblock™ NPJ03 treated nonwoven material shows excellent antiviral efficacy.


(7) Test performed according to Aerosol challenge test (ASTM F2101) with modifications to virus testing.
(8) 2013, ID number 798110-798116, Microbac Laboratories Inc. USA, Dr. Zhou. ASTM F2101 is NOT a simple antimicrobial test!The test mask is mounted and sealed within a test chamber. A nebulizer delivers an aerosol of the target virus inoculum to the upstream side of the mask. A collection dish placed below the mask downstream collects aerosol droplets that pass through the mask sample. The reduction in infectivity with and without mask is calculated as an indicator of effectiveness.
(9) Difference in log reduction of Δ=1 indicates 10x and Δ=2 indicates 100x
(10) 2020, 2020FM 03839R06 test report, “ISO 18184 – Determination of antiviral activity of textile products”, Guandong Detection Center of Microbiology, China
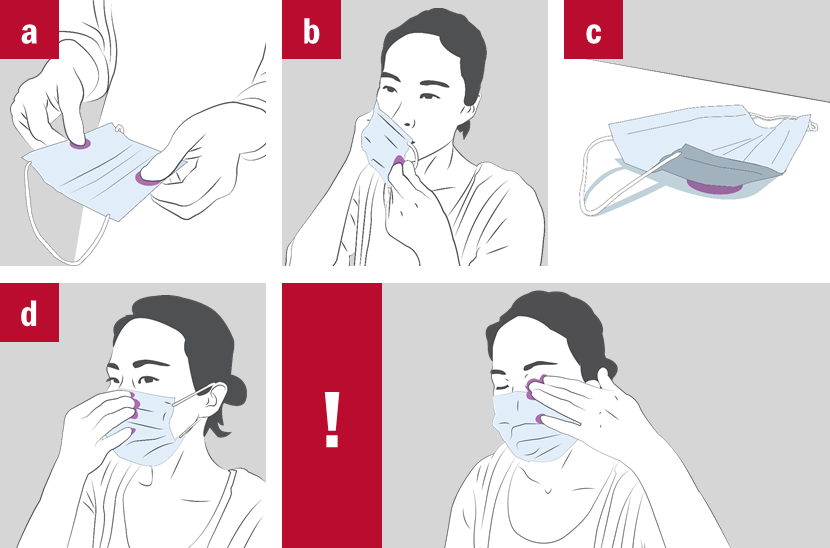
A potential vector: textile surface
Face masks are supposed to protect the wearer and/or the others but they are also a potential vector for viruses and bacteria!
During, before and after use, there is a risk of transferring pathogen to and picking up pathogen from the surface of the face masks:
a) When picking it up
b) When putting it on or taking off
c) When disposing it unsafely/leaving it lying around
d) When touching it while wearing or for adjustment
And there is always the risk of contracting the virus through touching the face after touching the contaminated surface of the mask or other surfaces contaminated by the mask.
Effective in minutes
HeiQ Viroblock’s rapid mode of action makes this technology one-of-a-kind to destroy harmful enveloped viruses.
It is a unique combination of HeiQ’s advanced silver technology and a vesicle technology as a booster. Silver emits silver-ions to inhibit bacteria on the fabric surface. The vesicle technology depletes the viral membrane thus silver can work more efficiently and rapidly to attack the core of the virus.
HeiQ Viroblock™ is designed for a wide range of applications:
Protective medical fabrics, e.g. used for face masks, air filters, medical gowns, curtains, drapes; clothing, upholstery, mattresses; products of all fiber types (especially nonwovens) can benefit from this innovative fabric treatment.

Apparel

Home textiles

Medical nonwoven

Mattresses
Breakthrough combination of HeiQ’s silver and vesicle technology
HeiQ Viroblock NPJ03
Combination of HeiQ’s silver & vesicle technology: the silver technology attracts the oppositely charged viruses and binds permanently to their sulfur groups. Subsequently, the fatty spherical vesicle technology (Liposomes) helps to deplete the viral membrane of its cholesterol content in minutes, therefore the silver can destroy the virus rapidly.
End use:
Protective medical fabrics, e.g. used for face masks, air filters, medical gowns, curtains, drapes; clothing, upholstery, mattresses
For all fiber types, especially nonwovens
![]()
![]()

OEKO-TEX SUITED
Test methods
HeiQ recommends the below test methods for the determination of the antibacterial and antiviral efficacy of treated textiles. The testing of the treated fabric is required for mill validation and for trademark licensing.
Antibacterial test
– Batch approval by mill:
- AATCC 1471 as qualitative antimicrobial test with Staphylococcus aureus
Antibacterial test
– approval by HeiQ:
- ISO 207431, 2 or JIS19021 quantitative antimicrobial test with Staph. A.
- ASTM 21491, 2 with Staph. A. for hydrophobic fabrics
- HeiQ Yogurt Bac test2
Antiviral test:
- ISO 181841 with the enveloped virus H3N2 or H1N1 or Coronavirus type
(HeiQ can recommend testing laboratories, but does not perform antiviral tests)
1 Reputable laboratory of customer‘s choice (third party) – Quality Control only
2 HeiQ’s Swiss contract lab Microbe Investigations AG (MIS) – Quality Validation free of charge / Quality Control at cost
Frequently Asked Questions
HeiQ Viroblock™ NPJ03 is a unique combination of our registered silver technology for antiviral and antibacterial effect and our vesicle technology as a booster.
The silver technology attracts the oppositely charged viruses and binds permanently to their sulfur groups. Subsequently, the fatty spherical vesicle technology (Liposomes) helps to deplete the viral membrane of its cholesterol content in minutes. Therefore, the silver can destroy the virus rapidly.
Yes. HeiQ Viroblock™ NPJ03, lasts up to 30 washes at 60°C.
Study shows that SARS-CoV, a similar virus to SARS-CoV-2 (the virus that causes COVID-19) can survive 2 days on a textile surface. While there hasn’t been such complete study on SARS-CoV-2 yet, one cannot rule out the same persistence of this virus ton textiles. So you might be bringing the virus home on your textile or your face mask, and there’s always a risk that you touch the textile and then don’t wash your hands properly before you change your outdoor clothing to indoor clothing, for instance. Then, there’s always the risk that you’ll touch your face and get infected that way. That’s exactly the risk that we want to mitigate. We want to make the textile surface such that the virus and bacteria are deactivated immediately in a short time – and we’re talking minutes.
We created a technology concept previously, but business disappeared quickly after the SARS crisis was over. The technology we had developed was shelved but not forgotten. So, when the 2019 novel coronavirus broke out, we were able to react very quickly. It took us three months to get everything back up and running as a supply chain, but we were able to respond.
COVID-19 is the name of the disease caused by the virus SARS-CoV-2.
While it is neither a medicine nor a vaccine, therefore should in no way claim efficacy against the disease, HeiQ Viroblock NPJ03 treated fabric has been tested effective against the SARS-CoV-2 virus. Tests conducted by an independent institute showed that treated fabric achieved 99.99% reduction of the virus after 30 minutes.
Our technology has also been tested against the human coronavirus 229E, Influenza types H1N1, H5N1, H7N9, and Respiratory Syncytial Virus (RSV) and demonstrated significantly improved reduction of virus infectivity.
M. J. (2020) Report on “Viral Stability and Persistence of SARS-CoV-2 on Treated Material”. Doherty Institute for Infection and Immunity, Australia.
Yes.
A human patch dermatological testing was similarly performed, which demonstrated a perfect skin compatibility.
HeiQ offers service for antibacterial testing as usual, for antiviral testing (ISO 18184) the costing and organization of third-party laboratory is at customer’s responsibility. Contact HeiQ for recommendations on testing laboratories which can perform the recommended ISO 18184 tests method.
No impairment of handfeel has been seen.
HeiQ Viroblock™ NPJ03 can be applied to woven, knit and nonwoven, cellulosic, synthetics and their blends. End use of nonwoven fabrics would be most suitable for the intended antiviral performance.
Yes, the dosage amount influences the effectiveness. The gradient is as following: 10% for maximum performance, 8.5% for strong performance, 7% good performance.
Recommended applications are 10% for critical applications like face masks and disposable medical nonwovens. 7-10% for less critical textile applications such as daily garments and home fabrics.
Discover more about this technology!
Unlock the power of Ingredient Branding with HeiQ
Check out our exciting Marketing Ideas package or contact marketing@heiq.com

Ingredient Branding with HeiQ
Check out our exciting Marketing Ideas package or contact marketing@heiq.com

